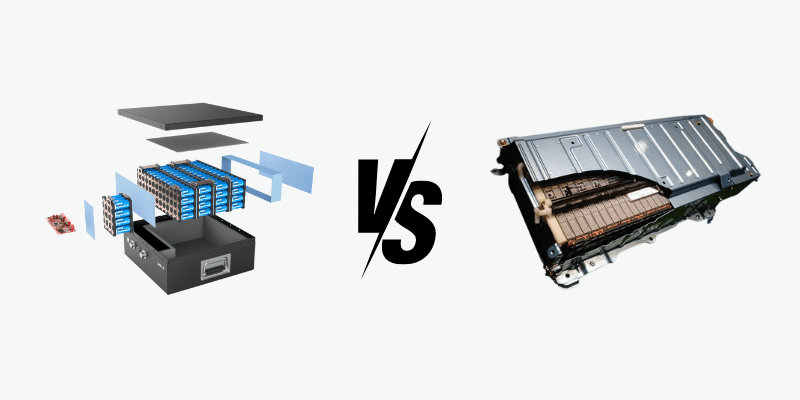
Elektriske køretøjsbatterier og lithium-ion-batterier til energilagring har forskellige krav, på trods af at begge er lithium-ion. At forstå deres forskelle kan hjælpe med at bestemme det bedste batteri til dine behov, da der ikke er nogen-størrelse-passer-alle-tilgang.
Hvad er et lithium-ion-batteri til energilagring lavet af?
Energilagringsapplikationer kræver batterier, der er meget pålidelige, langvarige og sikre.
Vores løsning bruger et lithiumjernphosphat (LFP) katode, som er ideel til disse krav. Den mindre interaktion mellem LFP -katoden og elektrolytten bidrager markant til dens overlegne cyklusliv og termisk stabilitet. Dette minimerer risikoen for termisk flugt, en potentielt farlig tilstand i nogle lithium-ion-batterier.
Mens LFP tilbyder enestående pålidelighed og sikkerhed, har den lavere energitæthed sammenlignet med andre lithium-ion-kemister som Lithium Cobaltoxid (LCO) eller Lithium Nickel Mangan Cobaltoxid (NMC). Derfor er LFP -batterier typisk tungere for en given mængde energi, hvilket er mindre ideelt til elektriske køretøjer.

Hvad er et lithiumionbatteri til elektriske køretøjer lavet af?
Elektriske køretøjer har brug for batterier med høj energi-densitet for at drive både bilen og dens ombord.
De fleste elbiler bruger lithium-ion-batterier med nikkel- og koboltkatoder, kaldet NMC batterier. Disse giver en tættere energikilde til fremdrift af køretøjer sammenlignet med LFP -batterier.
Den organiske flydende elektrolyt i NMC -batterier reagerer med ilt, især ved høje temperaturer. Denne reaktivitet kan føre til eksplosioner og andre potentielle problemer relateret til termisk løb.

Hvad er forskellene mellem lithiumionbatterier til energilagring og lithiumionbatterier til elektriske køretøjer?
LFP- og NMC -batterier er begge fremragende effektløsninger, men har vigtige forskelle, der gør dem velegnede til specifikke opgaver.
Katodemateriale
Lithium-ion-batterier bruger en katode til at generere strøm.
Energilagringsbatterier bruger LFP, mens elektriske køretøjsbatterier bruger NMC.
NMC -batterier har højere energitætheder, hvilket forbedrer accelerationen. LFP -batterier tilbyder overlegen energilagring sammenlignet med NMC.
Oxygenbinding
NMC- og LFP -batterier kræver begge en elektrolyt for at reagere med ilt. Imidlertid har NMC -batterier en løsere iltbinding, hvilket gør dem mere tilbøjelige til termisk løb og potentielle eksplosioner.
I modsætning hertil er LFP -batterier mindre tilbøjelige til at opleve termisk løb, hvilket gør dem til en mere sikker mulighed generelt.
Opladning & Udladning
NMC -batterier har højere effekttætheder end LFP -batterier, hvilket muliggør hurtigere opladning og udledning.
Batteri aldringsproces
NMC -batterier fungerer ved 3,7V, højere end 3,2V af LFP -batterier. Denne højere spænding fører til hurtigere nedbrydning, når batteriet ældes.
LFP batterier’ Lavere 3,2V -spænding giver større katodestabilitet, hvilket resulterer i længere batterilevetid.
Det større LFP -molekyle giver også mulighed for lettere ekspansion og sammentrækning under cykling, hvilket gør det muligt for LFP -batterier at modstå tusinder af cykler over deres levetid.
Koste
LFP-batterier er generelt mere omkostningseffektive pr. Cyklus, hvilket gør dem attraktive for langvarig omkostningseffektivitet.
NMC-batterier er dyrere på grund af deres katode, men kan være omkostningseffektive, hvor plads og vægt er begrænsninger takket være deres ydeevne og kompakte størrelse.
Ved hvilken spænding fungerer et lithium-ion-batteri til energilagring?
Lithium-ion-batterier designet til energilagring fungerer med 3,2 volt pr. Celle. Denne spænding matcher bly-syre-batterier, hvilket gør LFP-batterier egnede til 12, 24 eller 48-volt opbevaringssystemer.

Ved hvilken spænding fungerer et lithium -ionbatteri til elektriske køretøjer?
Elbilbatterier opererer med 3,7 volt pr. Celle med pakningsspændinger omkring 400 volt. Den højere spænding øger interaktioner mellem elektrolytten og katoden, hvilket giver mere strøm, men reducerer batteriets levetid. Denne afvejning er muligvis ikke ideel til stationær opbevaring, men den passer til behovene for at drive elektriske køretøjer.
Konklusion
Mens lithium-ion-batterier bruges til både energilagring og elektriske køretøjer, har deres specifikke krav ført til forskellige batterikemister.
Energilagringssystemer prioriterer pålidelighed, lang levetid og sikkerhed, hvilket gør batterier med lithiumjernphosphat (LFP) ideelle. Deres termiske stabilitet og cykluslivsdrag stationære applikationer.
Elektriske køretøjer kræver imidlertid høj energitæthed for at maksimere driving række og ydeevne, hvilket førte producenter til at favorisere lithium-ion-batterier med nikkel og koboltbaserede (NMC) katoder. Skønt lidt mindre stabile end LFP, kan NMC -batterier pakke mere energi i en mindre, lettere pakke – Kritisk for mobile applikationer.