Дискуссия между батареями сухих клеток и с влажными клетками значительна в технологии батареи. Понимание их различий важно для выбора правильного источника питания для различных приложений. Давайте рассмотрим различия и преимущества каждого типа.
Что такое батарея сухой ячейки?
Композиция и структура
Батарея сухого ячейки содержит несколько важных компонентов, которые генерируют электрическую энергию, в том числе:
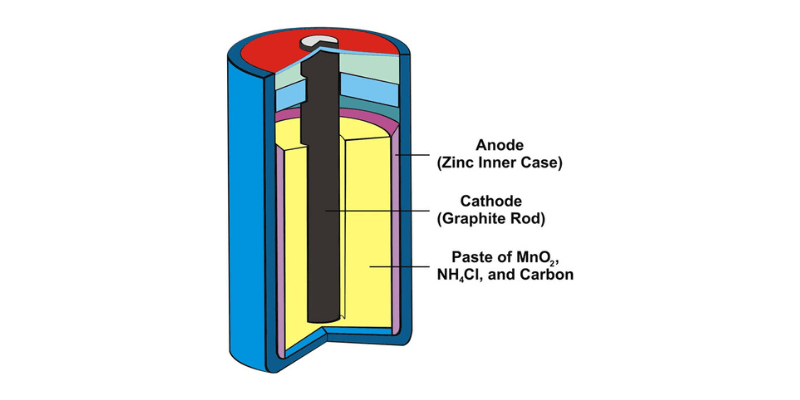
- Анод (отрицательный электрод): анод, обычно изготовленный из цинка, - это место, где происходит окисление, что приводит к потере электрона во время разряда батареи.
- Катод (положительный электрод): катод, изготовленный из углерода или графита, смешанный с диоксидом марганца, - это то, где происходит восстановление, получая электроны во время разряда.
- Электролит: в отличие от жидких электролитов, батареи сухих ячейки используют пасту электролит, часто смесь хлорида аммония и хлорида цинка, которая облегчает перенос ионов между анодом и катодом.
- Сепаратор: расположен между анод и катод, сепаратор, обычно изготовленный из бумаги или аналогичного материала, предотвращает прямой контакт и короткое замыкание, позволяя ионам проходить.
- Корпус: сборка помещена в герметичный корпус, обычно изготовленный из цинка или стали, который защищает внутренние компоненты и служит катодным терминалом для электрических соединений.
Преимущества и недостатки
Батареи сухого ячейки предлагают несколько преимуществ, которые делают их популярными для питания электронных устройств:
- Портативность: Сухие ячейки являются компактными и легкими, идеально подходят для портативных устройств.
- Безопасность: Они безопасны в использовании, так как им не хватает жидких электролитов, которые могут протекать.
- Длинный срок годности: Сухие клетки сохраняют заряд в течение длительных периодов, даже когда они не используются.
- Доступность: Они широко доступны и доступны.
- Простой в использовании: Не требуется специального обработки или технического обслуживания.
В то время как батареи сухих ячейки дают многочисленные преимущества, они также поставляются с определенными недостатками, о которых пользователи должны знать:
- Нерезарно: После истощения их нельзя заряжать и должны быть заменены.
- Более низкая плотность энергии: Сухие клетки хранят меньше энергии по сравнению с некоторыми другими Типы аккумуляторов.
- Воздействие на окружающую среду: Неправильное утилизация может нанести вред окружающей среде.
- Упадение напряжения: Их напряжение уменьшается при разряде, влияя на производительность устройства.
- Ограниченная продолжительность жизни: У них ограниченное количество циклов заряда.

Приложения
Сухие ячейки, портативные батареи, известные своими удобствами, безопасностью и надежностью, используются в различных применениях. Вот некоторые распространенные применения:
Бытовая электроника:
- Дистанционное управление: для телевизоров и кондиционеров.
- Портативные радиоприемники: для музыки или новостей на ходу.
- Фонарики: для освещения темных областей.
- Часы: для хронометража в таких устройствах, как настенные часы и будильники.
- Калькуляторы: для математических расчетов.
- Цифровые камеры: для питания функций камеры.
- Портативные CD/DVD -плееры: для воспроизведения аудио и видео.
- MP3 -плееры: для музыки прослушивание.
Игрушки и игры:
- Игрушки с дистанционным управлением: автомобили, самолеты и т. Д.
- Игрушки с аккумулятором: куклы, фигурки, электронные игры.
Аварийное оборудование:
- Аварийные огни & Радио: предоставьте свет и новости во время отключений.
Другие приложения:
- Медицинские устройства: электроси по слухам и мониторам.
- Научные инструменты & военная техника.

Что такое мокрый ячейка?
Композиция и структура
В мокрой ячейке компоненты погружаются в раствор жидкого электролита. Ключевые элементы включают:
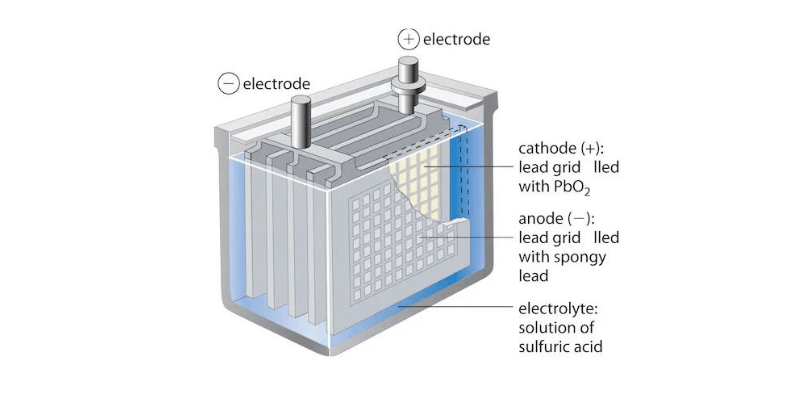
- Анод (отрицательный электрод): изготовлен из свинца (PB), он окисляется во время разряда и выпускает электроны.
- Катод (положительный электрод): состоит из диоксида свинца (PBO2), он получает электроны во время восстановления в катоде.
- Раствор электролита: в отличие от сухих клеток, батареи влажных клеток используют жидкую смесь серной кислоты (H2SO4) и воды (H2O) для облегчения потока ионов.
- Сепаратор: Пористый сепаратор предотвращает прямой контакт между анодом и катодом, позволяя ионному проходу избежать короткого замыкания.
- Корпус: прочный пластиковый или резиновый корпус содержит сборку, удерживая раствор электролита и обеспечивая конструктивную поддержку.
Преимущества и недостатки
Преимущества влажных ячеек:
- Высокая плотность мощности: Влажные ячейки, особенно свинцовые, обеспечивают высокую мощность для применений, нуждающихся в внезапных энергетических всплесках, таких как запуск автомобильного двигателя.
- Бюджетный: Как правило, они более доступны, чем другие технологии аккумуляторов по часовым часам.
- Длинная велосипедная жизнь: При правильном обслуживании батареи влажных ячеек могут длиться много лет, особенно в применении глубокого цикла.
- Racyclity: Ведущие аккумуляторы очень пригодны для переработки, что минимизирует воздействие на окружающую среду.
- Широкая доступность: Они легко доступны и заменяются.
Недостатки влажных клеточных батарей:
- Обслуживание: Влажные ячейки нуждаются в регулярном техническом обслуживании, включая проверку и добавление дистиллированной воды для потерянного электролита.
- Тяжелый и громоздкий: Они больше и тяжелее, чем другие типы аккумуляторов из -за их жидкого электролита.
- Кислотный электролит: Кислотный жидкий электролит опасен, если он не обращается с употреблением.
- Ограниченный велосипедный срок службы: У них долгий срок службы, но со временем ухудшается, особенно с глубокие разряды.
- Экологические проблемы: Неправильное утилизация свинцовых аккумуляторов может нанести вред окружающей среде.

Приложения
Влажные ячейки, особенно свинцово-кислотные батареи, популярны благодаря высокой плотности мощности и низкой стоимости. Общие приложения включают:
Автомобильная промышленность:
- Автомобильные батареи: запустите двигатели и электрические системы питания.
- Мотоциклетные батареи: системы силового зажигания и компоненты.
- Аккумуляторы грузовиков: больше автомобильных батарей для более тяжелых грузов.
Бесперебойные источники питания (UPS):
- Серверные комнаты: резервная мощность во время отключений.
- Главная/офисные ИБП: защитите чувствительное оборудование от скачков и отключений.
Промышленное применение:
- Аккумуляторные батареи: электрические вилочные погрузчики на складах.
- Хранение солнечной энергии: хранить избыточную солнечную энергию для последующего использования.
- Аварийное освещение: резервная мощность для систем аварийного освещения.
Морские приложения:
- Батареи лодок: морская электроника, трюмные насосы и т. Д.
- Подводные батареи: сосуды продвигают и поддерживают бортовые системы.
Другие приложения:
- Гольф -тележки: электрические гольф -тележки.
- Медицинское оборудование: питание для снабжения на такие устройства, как вентиляторы.
- Телекоммуникации: резервная мощность для башен.
В чем разница между батареями сухих и влажных клеток?
Основное различие между батареями сухих клеток и влажных клеток-это тип используемого электролита:
Электролит
- Сухие ячейки: пастообразное вещество с достаточным количеством влаги для проводимости, будучи достаточно прочной, чтобы предотвратить утечку.
- Влажные клетки: жидкий раствор, обычно кислый или щелочный.
Портативность
- Батареи сухой ячейки: очень портативные из -за их твердого электролита.
- Влажные ячейки: менее портативные из -за жидкого электролита.
Обслуживание
- Батареи сухой ячейки: требуется минимальное обслуживание.
- Влажные ячейки: требует регулярного технического обслуживания, например, проверка и добавление электролита.
Безопасность
- Батареи сухой ячейки: безопаснее, чем влажные ячейки, потому что они менее подвержены утечке электролита. Иммобилизованная электролитная паста сводит к минимуму риски аварии.
- Влажные ячейки: они могут быть опасными из -за их коррозионного раствора электролита, который представляет риски безопасности, если они не обработаны или повреждены.
| Особенность | Батарея сухого клеток | Батарея с влажным клетками |
| Электролит | Пастообразное вещество | Жидкий раствор |
| Портативность | Высоко портативно | Менее портативный |
| Обслуживание | Минимальное обслуживание | Регулярное обслуживание |
| Безопасность | Безопаснее | Менее безопасно |
Часто задаваемые вопросы
Что лучше, сухая ячейка или батарея мокрой ячейки?
Выбор между сухой ячейкой и батареей влажной ячейки зависит от применения и ваших приоритетов.
Автомобильные батареи влажные или сухие ячейки?
Автомобильные батареи представляют собой в основном влажные ячейки, использующие жидкий электролит, такой как серная кислота для химических реакций, которые генерируют электрическую энергию. Хотя новые технологии, такие как батареи AGM (поглощенный стеклянный коврик), маркируются “запечатанный” или “без технического обслуживания,” Они по -прежнему содержат жидкий электролит, поглощенный в коврике, классифицируя их как мокрые батареи.
Литийная батарея сухой или влажной?
Литиевые батареи классифицируются как сухие ячейки. Несмотря на то, что они содержат жидкий электролит, он удерживается внутри пористого сепаратора, предотвращая свободный поток. Эта конструкция повышает переносимость и уменьшает утечки по сравнению с традиционными влажными ячейками.