Het debat tussen droge-cel- en natte-celbatterijen is belangrijk in de batterijtechnologie. Het begrijpen van hun verschillen is belangrijk bij het kiezen van de juiste stroombron voor verschillende toepassingen. Laten we de verschillen en voordelen van elk type onderzoeken.
Wat is een droge celbatterij?
Samenstelling en structuur
Een drogecelbatterij bevat verschillende essentiële componenten die elektrische energie opwekken, waaronder:
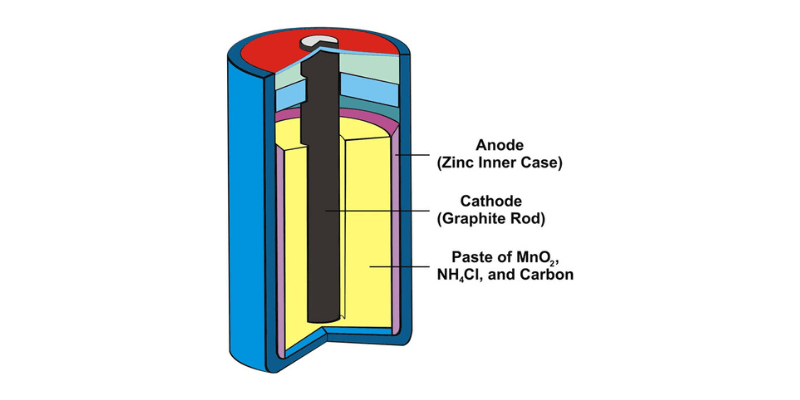
- Anode (negatieve elektrode): In de anode, meestal gemaakt van zink, vindt oxidatie plaats, wat resulteert in elektronenverlies tijdens het ontladen van de batterij.
- Kathode (positieve elektrode): De kathode, gemaakt van koolstof of grafiet gemengd met mangaandioxide, is waar reductie plaatsvindt, waarbij tijdens de ontlading elektronen worden verkregen.
- Elektrolyt: In tegenstelling tot vloeibare elektrolyten gebruiken droge celbatterijen een pasta elektrolyt, vaak een mengsel van ammoniumchloride en zinkchloride, dat de ionenoverdracht tussen de anode en kathode vergemakkelijkt.
- Separator: Geplaatst tussen de anode en kathode, de separator, meestal gemaakt van papier of een soortgelijk materiaal, voorkomt direct contact en kortsluiting, terwijl ionen er doorheen kunnen bewegen.
- Behuizing: Het geheel is gehuisvest in een afgedichte behuizing, meestal gemaakt van zink of staal, die de interne componenten beschermt en dient als kathode-aansluiting voor elektrische verbindingen.
Voordelen en nadelen
Droge celbatterijen bieden verschillende voordelen die ze populair maken voor het voeden van elektronische apparaten:
- Draagbaarheid: Droge cellen zijn compact en lichtgewicht, ideaal voor draagbare apparaten.
- Veiligheid: Ze zijn veilig in gebruik, omdat ze geen vloeibare elektrolyten bevatten die kunnen lekken.
- Lange houdbaarheid: Droge cellen behouden hun lading gedurende langere perioden, zelfs als ze niet worden gebruikt.
- Beschikbaarheid: Ze zijn breed toegankelijk en betaalbaar.
- Gemakkelijk te gebruiken: Er is geen speciale behandeling of onderhoud nodig.
Hoewel drogecelbatterijen talloze voordelen bieden, brengen ze ook bepaalde nadelen met zich mee waar gebruikers zich bewust van moeten zijn:
- Niet-oplaadbaar: Als ze eenmaal leeg zijn, kunnen ze niet meer worden opgeladen en moeten ze worden vervangen.
- Lagere energiedichtheid: Droge cellen slaan minder energie op dan andere batterij typen.
- Milieu-impact: Onjuiste verwijdering kan schadelijk zijn voor het milieu.
- Spanningsdaling: Hun spanning neemt af naarmate ze ontladen, wat de prestaties van het apparaat beïnvloedt.
- Beperkte levensduur: Ze hebben een beperkt aantal laad-ontlaadcycli.

Toepassingen
Droge cellen, draagbare batterijen die bekend staan om hun gemak, veiligheid en betrouwbaarheid, worden in verschillende toepassingen gebruikt. Hier zijn enkele veelvoorkomende toepassingen:
Consumentenelektronica:
- Afstandsbedieningen: voor tv's en airconditioners.
- Draagbare radio's: voor muziek of nieuws onderweg.
- Zaklampen: Voor het verlichten van donkere gebieden.
- Klokken: voor tijdregistratie in apparaten zoals wandklokken en alarmen.
- Rekenmachines: voor wiskundige berekeningen.
- Digitale camera's: Om camerafuncties aan te drijven.
- Draagbare cd-/dvd-spelers: voor het afspelen van audio en video.
- MP3-spelers: voor het luisteren naar muziek.
Speelgoed en spellen:
- Op afstand bestuurbaar speelgoed: auto's, vliegtuigen, enz.
- Speelgoed op batterijen: poppen, actiefiguren, elektronische spellen.
Nooduitrusting:
- Noodverlichting & radio's: zorg voor licht en nieuws tijdens storingen.
Andere toepassingen:
- Medische apparaten: elektrische hoortoestellen en monitoren.
- Wetenschappelijke instrumenten & militaire uitrusting.

Wat is een natte celbatterij?
Samenstelling en structuur
In een natte celbatterij worden componenten ondergedompeld in een vloeibare elektrolytoplossing. Belangrijke elementen zijn onder meer:
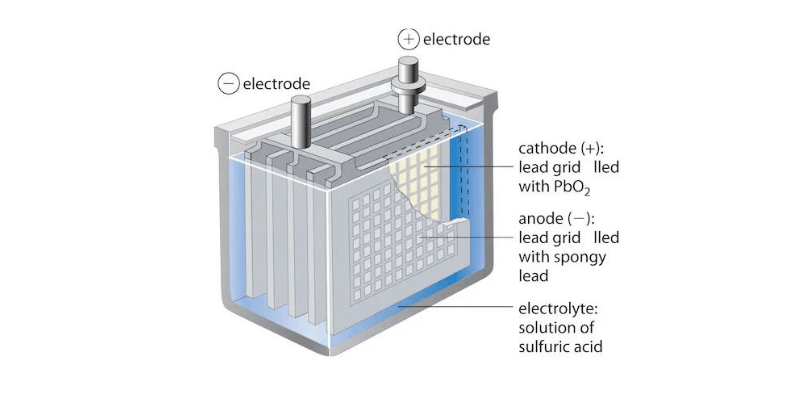
- Anode (negatieve elektrode): Gemaakt van lood (Pb), oxideert tijdens ontlading en geeft elektronen vrij.
- Kathode (positieve elektrode): Samengesteld uit looddioxide (PbO2), krijgt het elektronen tijdens de reductie aan de kathode.
- Elektrolytoplossing: In tegenstelling tot droge cellen gebruiken natte celbatterijen een vloeibaar mengsel van zwavelzuur (H2SO4) en water (H2O) om de ionenstroom te vergemakkelijken.
- Separator: Een poreuze separator voorkomt direct contact tussen de anode en kathode, terwijl ionendoorgang mogelijk is om kortsluiting te voorkomen.
- Behuizing: Een duurzame behuizing van kunststof of rubber huisvest het geheel, bevat de elektrolytoplossing en biedt structurele ondersteuning.
Voordelen en nadelen
Voordelen van natte celbatterijen:
- Hoge vermogensdichtheid: Natte celbatterijen, vooral loodzuurbatterijen, bieden een hoog uitgangsvermogen voor toepassingen die plotselinge energiestoten nodig hebben, zoals het starten van een automotor.
- Lage kosten: Ze zijn over het algemeen goedkoper dan andere batterijtechnologieën per wattuur.
- Lange levensduur: Met goed onderhoud kunnen natte celbatterijen vele jaren meegaan, vooral in deep-cycle-toepassingen.
- Recycleerbaarheid: Loodzuurbatterijen zijn zeer recyclebaar, waardoor de impact op het milieu tot een minimum wordt beperkt.
- Brede beschikbaarheid: Ze zijn gemakkelijk verkrijgbaar en vervangbaar.
Nadelen van natte celbatterijen:
- Onderhoud: Natte celbatterijen hebben regelmatig onderhoud nodig, inclusief het controleren en toevoegen van gedestilleerd water op verloren elektrolyt.
- Zwaar en omvangrijk: Ze zijn groter en zwaarder dan andere batterijtypen vanwege hun vloeibare elektrolyt.
- Zure elektrolyt: De zure vloeibare elektrolyt is gevaarlijk bij verkeerd gebruik.
- Beperkte levensduur: Ze hebben een lange levensduur, maar gaan na verloop van tijd achteruit, vooral met diepe ontladingen.
- Milieuproblemen: Een onjuiste verwijdering van loodzuuraccu’s kan schadelijk zijn voor het milieu.

Toepassingen
Natte celbatterijen, met name loodzuurbatterijen, zijn populair vanwege hun hoge vermogensdichtheid en lage kosten. Veel voorkomende toepassingen zijn onder meer:
Auto-industrie:
- Autoaccu's: Start motoren en voed elektrische systemen.
- Motoraccu's: elektrische ontstekingssystemen en componenten.
- Vrachtwagenaccu's: Groter dan autoaccu's voor zwaardere ladingen.
Ononderbroken voedingen (UPS):
- Serverruimten: back-upstroom tijdens storingen.
- UPS voor thuis/kantoor: bescherm gevoelige apparatuur tegen spanningspieken en stroomuitval.
Industriële toepassingen:
- Heftruckbatterijen: elektrische vorkheftrucks in magazijnen van stroom voorzien.
- Opslag van zonne-energie: opslag van overtollige zonne-energie voor later gebruik.
- Noodverlichting: Back-upstroom voor noodverlichtingssystemen.
Mariene toepassingen:
- Bootbatterijen: scheepselektronica, lenspompen, enz.
- Onderzeese batterijen: drijven schepen aan en ondersteunen boordsystemen.
Andere toepassingen:
- Golfkarretjes: elektrische golfkarretjes.
- Medische apparatuur: Voorzie apparaten zoals ventilatoren van stroom.
- Telecommunicatie: back-upstroom voor torens.
Wat is het verschil tussen droge cel- en natte celbatterijen?
Het belangrijkste verschil tussen droge cel- en natte celbatterijen is het type elektrolyt dat wordt gebruikt:
Elektrolyt
- Droge celbatterijen: een pasta-achtige substantie met voldoende vocht voor geleiding en tegelijkertijd stevig genoeg om lekkage te voorkomen.
- Natte celbatterijen: een vloeibare oplossing, meestal zuur of alkalisch.
Draagbaarheid
- Droge celbatterijen: zeer draagbaar dankzij hun vaste elektrolyt.
- Natte celbatterijen: minder draagbaar vanwege de vloeibare elektrolyt.
Onderhoud
- Droge celbatterijen: minimaal onderhoud vereist.
- Natte celbatterijen: Vereist regelmatig onderhoud, zoals het controleren en toevoegen van elektrolyt.
Veiligheid
- Droge celbatterijen: Veiliger dan natte celbatterijen omdat ze minder gevoelig zijn voor elektrolytlekkage. De geïmmobiliseerde elektrolytpasta minimaliseert het risico op ongevallen.
- Natte celbatterijen: ze kunnen gevaarlijk zijn vanwege hun corrosieve elektrolytoplossing, die veiligheidsrisico's met zich meebrengt als ze verkeerd worden gebruikt of beschadigd.
| Functie | Droge celbatterij | Natte celbatterij |
| Elektrolyt | Pasta-achtige substantie | Vloeibare oplossing |
| Draagbaarheid | Zeer draagbaar | Minder draagbaar |
| Onderhoud | Minimaal onderhoud | Regelmatig onderhoud |
| Veiligheid | Veiliger | Minder veilig |
Veelgestelde vragen
Wat is beter: een droge cel of een natte cel batterij?
De keuze tussen een droge cel- en een natte celbatterij hangt af van de toepassing en uw prioriteiten.
Zijn autoaccu's natte of droge cellen?
Autobatterijen zijn voornamelijk natte celbatterijen, die een vloeibare elektrolyt zoals zwavelzuur gebruiken voor chemische reacties die elektrische energie opwekken. Hoewel nieuwere technologieën, zoals AGM-batterijen (Absorbed Glass Mat), zijn gelabeld “verzegeld” of “onderhoudsvrij,” ze bevatten nog steeds een vloeibare elektrolyt die in een mat is geabsorbeerd, waardoor ze worden geclassificeerd als natte celbatterijen.
Is een lithiumbatterij droog of nat?
Lithiumbatterijen worden geclassificeerd als drogecelbatterijen. Hoewel ze een vloeibare elektrolyt bevatten, wordt deze in een poreuze afscheider vastgehouden, waardoor vrije doorstroming wordt voorkomen. Dit ontwerp verbetert de draagbaarheid en vermindert lekken in vergelijking met traditionele natte celbatterijen.