Hvis du nettopp har lært om anoder og katoder, er du ikke alene. Disse vilkårene er vanligvis bare relevante ved arbeid med varmtvannsberedere eller kjøretøybatterier. Denne artikkelen forklarer forskjellen i klarspråk. Vi vil diskutere hva anoder og katoder er, hvordan de er produsert og hvordan de fungerer.
Hva er batterianoder og katoder?
Anoder og katoder er de essensielle komponentene i batterier. De er elektrodene der elektrokjemiske reaksjoner oppstår, som gjør det mulig for batterier å lagre og frigjøre elektrisk energi.
Katoden er den positive elektroden hvor reduksjon skjer, mens anoden er den negative elektroden hvor oksidasjon finner sted.
Under batterilading strømmer elektroner fra katoden til anoden og lagrer energi for senere bruk.
Hvilke materialer brukes i anoder og katoder?
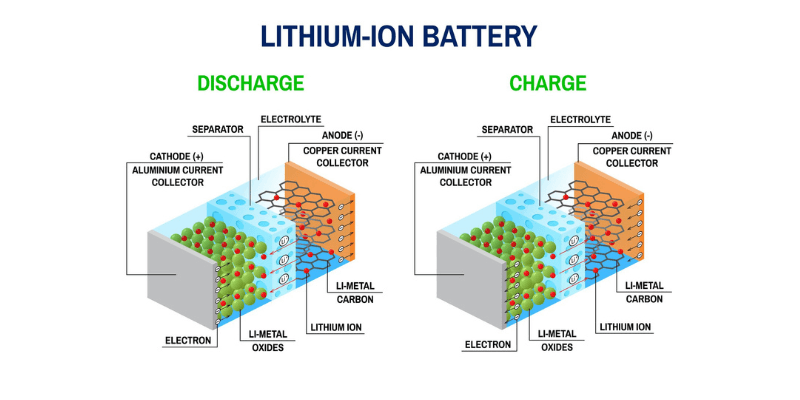
Katodeaktive materialer (CAM) er vanligvis metalloksider. For litiumionbatterier er de mest populære litiumjernfosfat (LiFePO4 eller LFP) og litiumnikkel mangan koboltoksid (LiNiMnCoO2 eller NMC). Disse materialene varierer i energitetthet, termisk stabilitet og kostnadseffektivitet.
Anode aktive materialer (AAM) er generelt karbonbaserte, som grafitt, silisium eller en kombinasjon. Grafitt er den vanligste på grunn av sin høye ledningsevne, lave kostnader og stabile struktur.
Hvordan anoder og katoder ble produsert
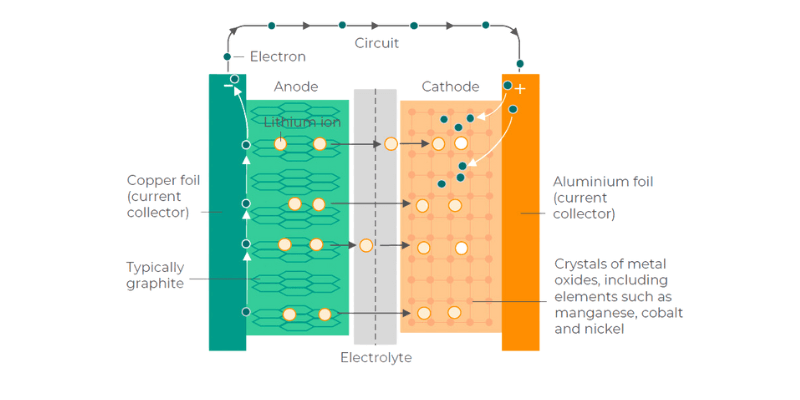
Fremstilling av batterianoder og katoder involverer vanligvis flere trinn.
Først syntetiseres de nødvendige katode- og anodematerialene til ønskede forbindelser. Disse materialene blir deretter malt til et pulver og blandet med bindemidler og løsemidler for å danne en slurry. Deretter blir slurryen belagt på metallfolier, tørket og komprimert gjennom valser. Til slutt kuttes de belagte anode- og katodefoliene til størrelse og kombineres med andre komponenter for å bygge en battericelle.
Hvordan fungerer anoder og katoder?
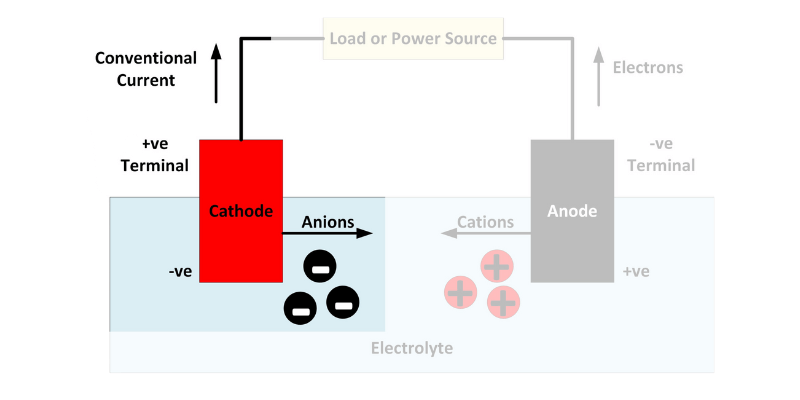
En anode er et oksiderende metall i et elektrolyttløsningsmiddel, som litium, som mister elektroner. Det eroderer sakte når elektroner beveger seg til katoden gjennom en leder. Når anoden er utladet, dør batteriet.
Katoden mottar elektroner fra anoden, og begge er nedsenket i en elektrolyttløsning. Elektrisitet går gjennom lederen fra negativ til positiv.
Hvordan skiller du en anode og katode fra hverandre?
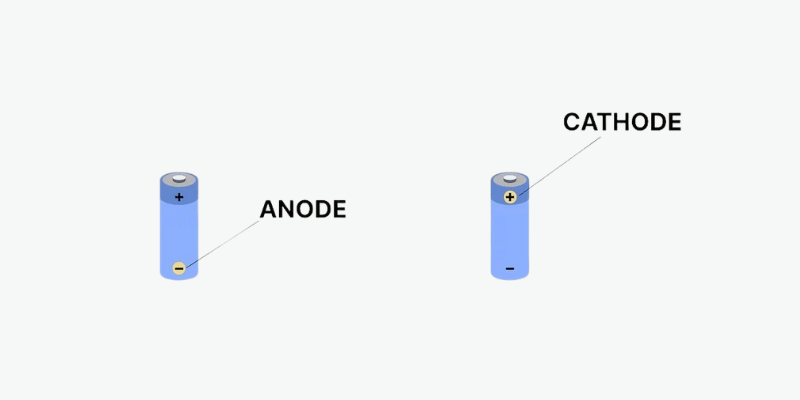
De fleste batterier har et pluss (+) og minus (-) tegn som indikerer anoden og katoden. Minustegnet refererer til anoden, som er den negative elektroden som mister elektroner. Plusstegnet refererer til katoden, den positive elektroden som får elektroner.
Konklusjon
Hvis du eier en bil, bobil, båt eller liker å tulle, er det nyttig å forstå anoder og katoder. Disse begrepene kommer kanskje ikke opp i hverdagen, men de finnes i batteriene, varmtvannsberederen og andre vanlige enheter.
Relaterte artikler: