Ak ste sa práve dozvedeli o anódach a katódach, nie ste sami. Tieto pojmy sú zvyčajne relevantné iba pri práci na ohrievačoch vody alebo batériách vozidiel. Tento článok vysvetľuje rozdiel v jednoduchom jazyku. Budeme diskutovať o tom, čo sú anódy a katódy, ako sa vyrábajú a ako fungujú.
Čo sú anódy a katódy batérií?
Anódy a katódy sú základnými komponentmi batérií. Sú to elektródy, kde dochádza k elektrochemickým reakciám, ktoré umožňujú batériám uchovávať a uvoľňovať elektrickú energiu.
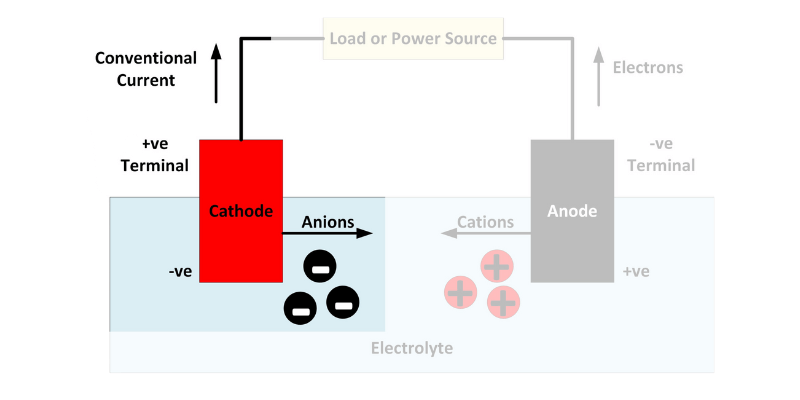
Katóda je kladná elektróda, kde dochádza k redukcii anóda je záporná elektróda kde prebieha oxidácia.
Počas nabíjania batérie prúdia elektróny z katódy na anódu a ukladajú energiu na neskoršie použitie.
Aké materiály sa používajú v anódach a katódach?
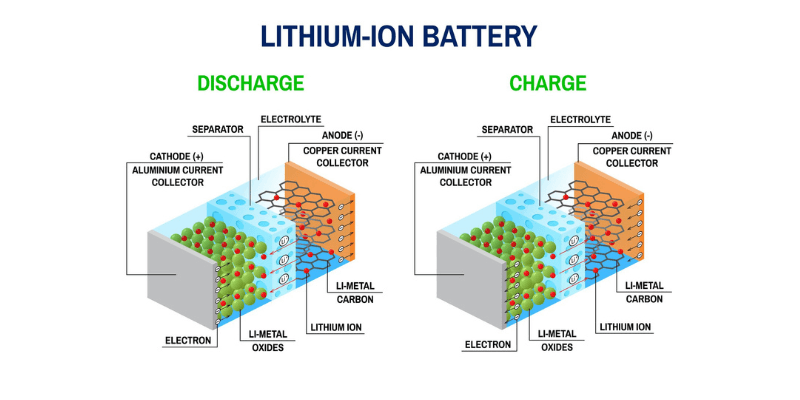
Katódové aktívne materiály (CAM) sú typicky oxidy kovov. Pre lítium-iónové batérie sú najobľúbenejšie fosforečnan lítno-železitý (LiFePO4 alebo LFP) a oxid lítium-nikel-mangán-kobaltnatý (LiNiMnCoO2 alebo NMC). Tieto materiály sa líšia hustota energietepelná stabilita a nákladová efektívnosť.
Anódové aktívne materiály (AAM) sú vo všeobecnosti na báze uhlíka, ako je grafit, kremík alebo ich kombinácie. Grafit je najbežnejší vďaka svojej vysokej vodivosti, nízkej cene a stabilnej štruktúre.
Ako sa vyrábajú anódy a katódy
Výroba batériových anód a katód zvyčajne zahŕňa niekoľko krokov.
Najprv sa potrebné materiály katódy a anódy syntetizujú do požadovaných zlúčenín. Tieto materiály sa potom rozomelú na prášok a zmiešajú so spojivami a rozpúšťadlami za vzniku kaše. Potom sa kaša nanesie na kovové fólie, vysuší a stlačí valcami. Nakoniec sa potiahnuté anódové a katódové fólie narežú na požadovanú veľkosť a kombinujú s inými komponentmi, aby sa vytvoril batériový článok.
Ako fungujú anódy a katódy?
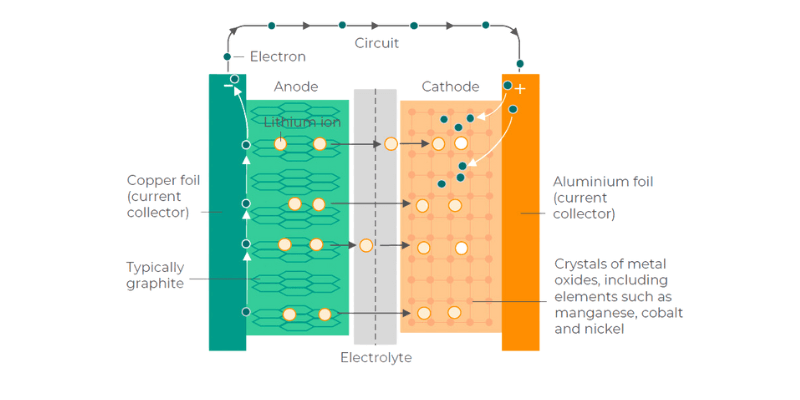
Anóda je oxidačný kov v rozpúšťadle elektrolytu, ako je lítium, ktorý stráca elektróny. Pomaly eroduje, keď sa elektróny pohybujú ku katóde cez vodič. Akonáhle je anóda vyčerpaná, batéria zomrie.
Katóda prijíma elektróny z anódy a obe sú ponorené v roztoku elektrolytu. Elektrina prechádza vodičom z negatívneho do pozitívneho.
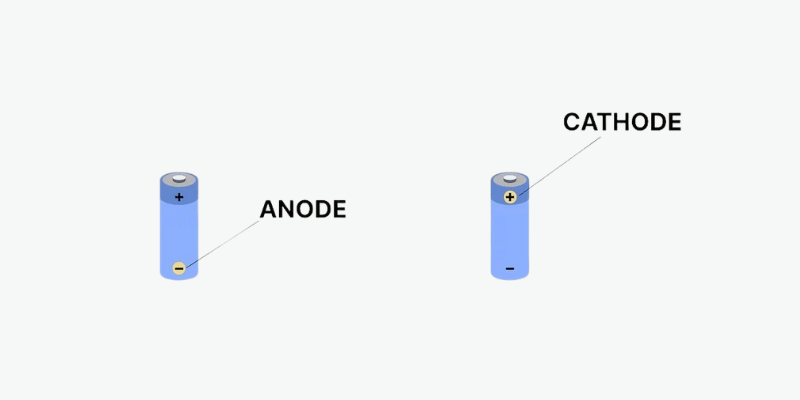
Ako rozlíšite anódu a katódu?
Väčšina batérií má znamienko plus (+) a mínus (-) označujúce anódu a katódu. Znamienko mínus sa týka anódy, čo je záporná elektróda, ktorá stráca elektróny. Znamienko plus sa vzťahuje na katódu, kladnú elektródu, ktorá získava elektróny.
Záver
Ak vlastníte auto, karavan, loď alebo radi kúrite, je užitočné porozumieť anódam a katódam. Tieto výrazy sa nemusia objaviť v každodennom živote, ale nachádzajú sa vo vašich batériách, ohrievači vody a iných bežných zariadeniach.
Súvisiace články: