Kluczowe wnioski:
- Częstość występowania i działanie: Baterie litowo-jonowe są szeroko stosowane ze względu na wysoką gęstość energii i brak efektu pamięci. Działają poprzez odwracalny ruch jonów litu pomiędzy katodą i anodą.
- Przyczyny awarii: Typowe przyczyny awarii akumulatorów obejmują odparowanie elektrolitu organicznego, topienie separatora, uwalnianie tlenu, niekontrolowane ładowanie, szybkie ładowanie w niskich temperaturach, całkowite rozładowanie i wady produkcyjne.
- Strategie zapobiegawcze: Zapewnienie trwałości baterii wymaga stosowania wysokiej jakości ogniw, efektywnej konstrukcji zestawu baterii i niezawodnego systemu zarządzania baterią (BMS).
- Znaczenie i funkcje BMS: BMS ma kluczowe znaczenie dla monitorowania napięcia, temperatury i równowagi ogniw. Powinien być zgodny z normami bezpieczeństwa, takimi jak UL 1642 i IEC 62133 dla ogniw oraz UL 991 lub UL 1998 dla oprogramowania BMS.
Baterie litowo-jonowe są wszędzie wokół nas, zasilając nasze smartfony, laptopy, pojazdy elektryczne i systemy magazynowania energii odnawialnej.
W tym poście omówimy podstawy tych akumulatorów, w tym sposób ich działania, zalety, najczęstsze przyczyny awarii i metody zapobiegania.
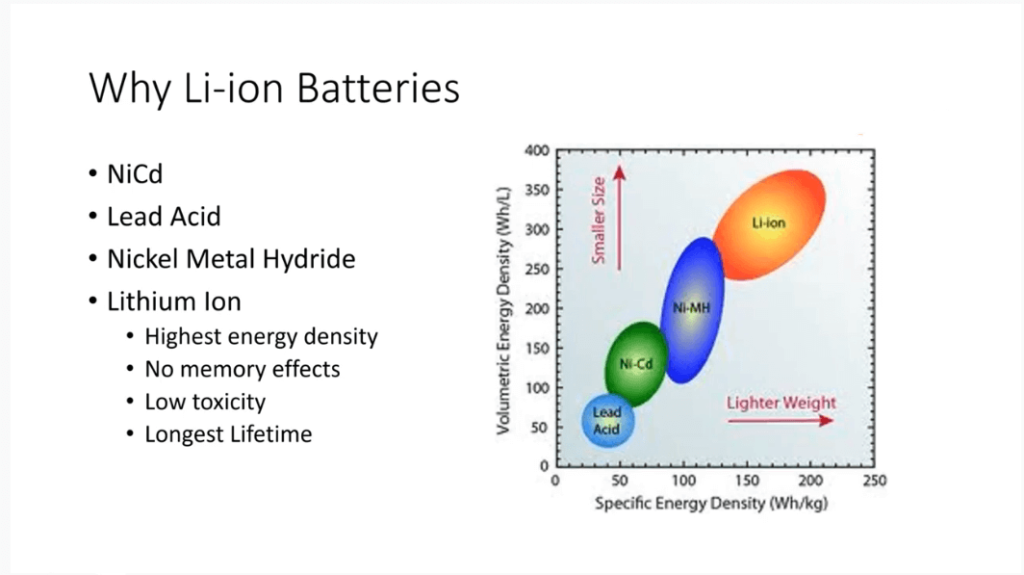
Dlaczego warto używać baterii litowo-jonowych?
Baterie litowo-jonowe stały się popularne ze względu na dużą gęstość energii. Są one lepsze od akumulatorów kwasowo-ołowiowych, niklowo-kadmowych i niklowo-metalowo-wodorkowych zarówno pod względem objętości, jak i gęstości energii w oparciu o masę.
Przejście z akumulatorów niklowo-kadmowych na akumulatory niklowo-metalowo-wodorkowe doprowadziło do powszechnego stosowania akumulatorów litowo-jonowych. Baterie te nie tylko oferują najwyższą gęstość energii, ale także nie posiadają efektu pamięci. Oznacza to, że na ich pojemność nie ma wpływu pełne lub częściowe ładowanie lub rozładowanie.
Poza tym akumulatory litowo-jonowe mają niską toksyczność. Zwłaszcza akumulatory litowo-żelazowo-fosforanowe, nie zawierają metali ciężkich, takich jak kobalt. Mają także dłuższą żywotność niż alternatywne chemikalia, zapewniając niezawodność w różnych zastosowaniach.
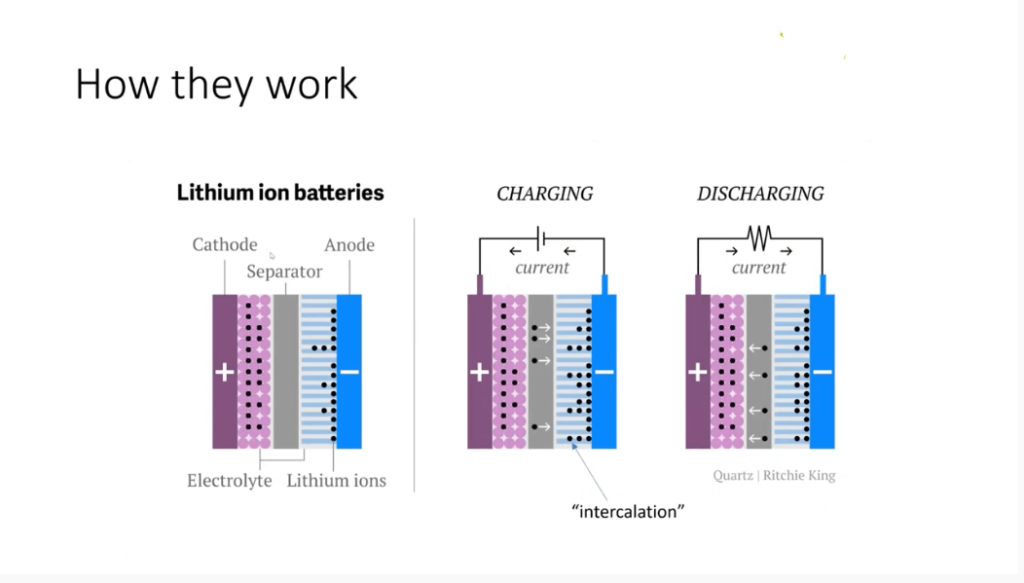
Jak działają baterie litowo-jonowe?
Aby zrozumieć zagrożenia związane z akumulatorami litowo-jonowymi, ważne jest zrozumienie ich działania. Jak każde ogniwo elektrochemiczne, bateria litowo-jonowa składa się z katody i anody. Katoda zwykle zawiera sól litu, taką jak tlenek litu lub fosforan litu, podczas gdy anoda jest zwykle wykonana z grafitu.
Podczas ładowania akumulatora litowo-jonowego jony litu (przedstawione przez czarne kropki) przemieszczają się z soli tlenku litu do anody grafitowej. Ruch ten, znany jako interkalacja, nie obejmuje bezpośredniej interakcji pomiędzy jonami i elektronami. Zamiast tego elektrony przepływają z katody do anody, gdzie reagują z węglem zawartym w graficie.
Warto wspomnieć, że w przeciwieństwie do akumulatorów litowo-metalowych, które nie nadają się do ponownego ładowania, akumulatory litowo-jonowe umożliwiają odwracalną interkalację jonów litu. Ta przełomowa innowacja przyznała Johnowi Goodenoughowi i Stanowi Winninghamowi Nagrodę Nobla w dziedzinie chemii. Jony litu ulegają dyfuzji przez organiczny płyn elektrolitowy, umożliwiając ich ruch tam i z powrotem pomiędzy anodą i katodą.
W następnej części przyjrzymy się bliżej elektrolitowi organicznemu i jego funkcji we wspomaganiu sprawnego działania akumulatorów litowo-jonowych.
LCO, LMO, NCA
Zacznijmy od omówienia katody i soli litu stosowanych zwykle w akumulatorach litowo-jonowych. Pierwszym z nich, który zbadamy, jest tlenek litu i kobaltu, który jest szeroko rozpowszechniony w laptopach, elektronarzędziach i telefonach komórkowych. Kiedy akumulator się rozładowuje, lit oddziela się od tlenku litu i kobaltu, uwalniając elektron, który przemieszcza się przez ładowarkę do anody. Ta procedura pozostawia tlenek kobaltu na katodzie.
Inną solą stosowaną jako materiał katodowy jest tlenek litowo-manganowy. Ten typ katody zastosowano w Nissanie Leaf i można go również znaleźć w różnych modelach Tesli, takich jak Model S, Model 3 i Model X.
Na koniec mamy tlenek litowo-niklowo-kobaltowy, który zapewnia najwyższą pojemność energetyczną na masę i objętość.
Przyczyny awarii akumulatora litowo-jonowego
Aby zapobiec awariom akumulatorów litowo-jonowych, należy zdawać sobie sprawę z czynników, które mogą prowadzić do takich problemów. Przyjrzyjmy się bliżej niektórym częstym przyczynom.
Odparowanie elektrolitu organicznego
Jeśli akumulator litowo-jonowy nagrzeje się zbyt mocno, znajdujący się w nim organiczny elektrolit może odparować. To parowanie zwiększa ciśnienie i temperaturę w komórce. W rezultacie bateria może się wybrzuszyć, wskazując na obecność niebezpiecznych warunków.
Topienie separatora
W akumulatorach litowo-jonowych zwykle stosuje się separator wykonany z polietylenu lub polipropylenu. Pod wpływem temperatury około 80 stopni Celsjusza (170–180 stopni Fahrenheita) separator ten może się stopić. Topienie separatora umożliwia kontakt anody i katody, co prowadzi do wewnętrznego zwarcia i generowania dodatkowego ciepła.
Uwalnianie tlenu i niekontrolowane reakcje
Kiedy akumulator litowo-jonowy osiąga wysokie temperatury, może zostać uwolniony tlen obecny w materiałach katod, takich jak tlenek litu, kobaltu, tlenek litu i manganu lub tlenek litu, niklu i kobaltu. Uwolniony tlen może reagować z odparowanym elektrolitem, powodując niekontrolowane reakcje chemiczne. Ciągłe zwarcie dodatkowo pogarsza sytuację, dlatego niezwykle ważne jest, aby szybko zaradzić temu problemowi.
Niekontrolowany ładunek
Przeładowanie akumulatora lub poddawanie go niekontrolowanemu ładowaniu może prowadzić do tworzenia się litu metalicznego na anodzie. Nadmiar elektronów łączy się z jonami litu, tworząc dendryty, które rosną przez elektrolit i katodę. Te dendryty mogą powodować wewnętrzne zwarcia, stwarzając poważne ryzyko.
Szybkie ładowanie i niskie temperatury
Ładowanie akumulatora bardzo wysokim prądem lub niskimi temperaturami może utrudniać przemieszczanie się jonów litu do anody. W rezultacie na anodzie może gromadzić się nadmiar elektronów, powodując platerowanie litem i potencjalne wewnętrzne zwarcia.
Całkowite rozładowanie
Unikaj całkowitego rozładowania ogniwa litowo-jonowego. Nadmierne rozładowanie może spowodować rozpuszczenie miedzianego kolektora na anodzie w elektrolicie. Podczas ładowania miedź może odtworzyć swoją pierwotną strukturę przypominającą folię. Może to prowadzić do pokrycia miedzią i wewnętrznego zwarcia.
Słaba produkcja komórek i zanieczyszczenie
Awarie akumulatorów litowo-jonowych mogą również wystąpić na skutek wad produkcyjnych lub obecności zanieczyszczeń podczas produkcji. Zanieczyszczenia te mogą wprowadzić zanieczyszczenia lub cząstki stałe do akumulatora, prowadząc do wewnętrznych zwarć lub niepożądanych reakcji, które przyspieszają spadek pojemności.
Rozumiejąc i eliminując te przyczyny awarii akumulatorów litowo-jonowych, możemy pracować nad zwiększeniem bezpieczeństwa, niezawodności i trwałości akumulatorów w różnych zastosowaniach.
Zapobieganie awariom baterii
Zapobieganie problemom w branży akumulatorów ma kluczowe znaczenie dla jej dalszego rozwoju i sukcesu. Aby skutecznie zminimalizować występowanie problemów, można podjąć trzy kluczowe kroki.
Przede wszystkim niezwykle ważne jest zapewnienie jakości ogniw akumulatorowych. Wraz z szybkim rozwojem branży powstały liczne zakłady produkujące ogniwa, szczególnie w Chinach. Ważne jest, aby starannie wybierać wysokiej jakości ogniwa, pochodzące od renomowanych producentów. Niektóre obiekty mogą poszczycić się najnowocześniejszymi, zaawansowanymi technologicznie, zautomatyzowanymi procesami, podczas gdy inne mogą nie spełniać tych samych standardów. Wybór jakości ogniw ma bezpośredni wpływ na ogólną wydajność i niezawodność.
Konstrukcja akumulatora również odgrywa kluczową rolę w zapobieganiu wypadkom. Zestawy akumulatorów składają się z wielu ogniw ułożonych szeregowo i równolegle, tworząc pożądane napięcie i wydajność prądową. Projektując opakowanie należy uwzględnić efektywne odprowadzenie ciepła na wypadek nieprzewidzianych zdarzeń. Zrozumienie, jak opakowanie zareaguje na potencjalne problemy z komórkami, ma kluczowe znaczenie dla utrzymania bezpieczeństwa. Co więcej, jeśli system wymaga dostarczania znacznych ilości prądu, zapewnienie wydajnej dystrybucji poprzez niezawodne styki i płytki drukowane staje się sprawą najwyższej wagi.
Podstawą tego wszystkiego jest system zarządzania baterią (BMS). To urządzenie pełni funkcję strażnika akumulatora, stale monitorując napięcia, prądy i temperatury, aby zapewnić działanie ogniw w bezpiecznych granicach. W każdym zestawie akumulatorów litowo-jonowych obecność zintegrowanego lub zewnętrznego systemu BMS ma kluczowe znaczenie dla zabezpieczenia ogniw. BMS nie tylko zapewnia bezpieczeństwo, ale także zwiększa żywotność akumulatorów. Biorąc pod uwagę, że akumulatory litowo-jonowe mogą znacznie wytrzymać konwencjonalne urządzenia magazynujące, konieczne staje się priorytetowe traktowanie ich ochrony w przypadku długotrwałego użytkowania.
Zapobieganie problemom w branży akumulatorów wymaga zwrócenia szczególnej uwagi na jakość ogniw, konstrukcję opakowania i wdrożenie niezawodnego systemu zarządzania akumulatorami. Te wspólne środki przyczyniają się do ogólnego bezpieczeństwa i trwałości akumulatorów litowo-jonowych, umożliwiając rozwój branży przy jednoczesnej minimalizacji potencjalnego ryzyka.
Znaczenie systemów zarządzania akumulatorami
System zarządzania akumulatorem (BMS) odgrywa kluczową rolę w monitorowaniu i kontrolowaniu napięć, prądów i temperatur akumulatora. Jego podstawową funkcją jest zapewnienie, że akumulator działa w bezpiecznych granicach. Jeśli BMS wykryje jakiekolwiek nieprawidłowości lub przekroczy limity ogniw, ma możliwość przerwania procesu ładowania lub rozładowywania.
Mówiąc prościej, BMS monitoruje parametry życiowe akumulatora. Stale sprawdza poziom napięcia, przepływ prądu i temperaturę, aby upewnić się, że wszystko działa prawidłowo. Jeśli wykryje jakiekolwiek problemy, takie jak nadmierne ciepło lub nieregularne napięcie, może podjąć działania w celu ochrony akumulatora.
Jednym z kluczowych zadań BMS jest zapobieganie przeładowaniu lub nadmiernemu rozładowaniu akumulatora. Przeładowanie może spowodować uszkodzenie ogniw akumulatora i skrócenie ich żywotności, natomiast nadmierne rozładowanie może prowadzić do pogorszenia wydajności. BMS dba o to, aby akumulator otrzymał odpowiednią ilość ładunku i zapobiega jego przepełnieniu lub nadmiernemu rozładowaniu.
Pomyśl o BMS jako o strażniku akumulatora. Jest zawsze na straży, gotowy do wkroczenia i ochrony akumulatora przed potencjalnymi uszkodzeniami. Monitorując i regulując parametry akumulatora, BMS pomaga wydłużyć jego ogólną żywotność i utrzymać optymalną wydajność.
Jakie funkcje powinny być obecne w BMS?
Chcemy podzielić się moją opinią na temat minimalnych wymagań stawianych BMS-owi, aby zapewnić ochronę i trwałość pakietu akumulatorów.
Po pierwsze, niezbędna jest ochrona napięcia. Ważne jest, aby zapobiegać przeładowaniu i nadmiernemu rozładowaniu akumulatora. Musimy utrzymać bezpieczny zakres napięcia, aby uniknąć uszkodzenia ogniw i zmaksymalizować ich żywotność. Nawiasem mówiąc, powinniśmy również rozważyć zapobieganie dostarczaniu przez pakiet prądów przekraczających jego pojemność, nie tylko na poziomie ogniwa, ale także całego pakietu.
Ochrona przed temperaturą to kolejny ważny aspekt. Zbyt wysoki wzrost temperatur może prowadzić do potencjalnych zagrożeń i awarii. Dlatego kluczowe znaczenie ma posiadanie mechanizmów monitorowania i kontrolowania wysokich temperatur. Podobnie ważne jest posiadanie zabezpieczenia ładowania w niskich temperaturach, aby zapobiec problemom, takim jak osadzanie się litu na anodzie z powodu zbyt niskich temperatur.
Co więcej, przydatną funkcją, choć nie jest to absolutnie konieczne, jest możliwość zrównoważenia ogniw w szeregu. Ogniwa połączone równolegle w naturalny sposób dzielą prąd i napięcie, ale ogniwa połączone szeregowo nie. Aby utrzymać jednolity stan naładowania (SOC) pomiędzy ogniwami, wymagany jest mechanizm równoważący lub możliwość dodatkowego współdzielenia prądu.
Na koniec, chociaż nie omawialiśmy konkretnych standardów dotyczących testów stron trzecich, warto wspomnieć, że istnieją standardy, które zewnętrzne laboratoria testowe mogą wykorzystać do oceny zgodności.
Standardy
Często pojawia się zamieszanie dotyczące różnych wykazów ogniw, pakietów akumulatorów i systemów zarządzania akumulatorami. Wyjaśnijmy trochę sprawę. Ogniwa litowo-jonowe można testować i wystawiać na liście zgodnie z normami UL 1642 lub IEC 62133.
Z drugiej strony akumulatory mają swoje własne wykazy. Mogą być wymienione w ramach norm UL 2050 lub UL 1973, przy czym w obu przypadkach wymagana jest zgodność z normą UL 1642. Należy zauważyć, że sama norma UL 1642 nie jest listą pakietów, ale raczej warunkiem wstępnym dla tych list pakietów.
Próbując stworzyć listę, która miałaby zastosowanie zarówno do ogniw, jak i pakietów, IEC wprowadziła normę IEC 62133. Warto jednak wspomnieć, że systemy zarządzania akumulatorami (BMS) również mają swoje własne, osobne wykazy.
W przypadku sprzętu BMS może być zgodny z normą UL 991, natomiast w przypadku oprogramowania może być zgodny z normą UL 1998 lub IEC 60730-1. Należy pamiętać, że wymagania UL 991 i UL 1998 nie są warunkiem wstępnym umieszczenia na liście UL 2054 lub UL 1973.
Jeśli jednak Twój system BMS nie jest zgodny z tymi normami, konieczne będzie przeprowadzenie testów w warunkach usterek, aby mieć pewność, że nawet w przypadku awarii nie powstanie niebezpieczna sytuacja.
Należy pamiętać, że nie jest to wyczerpująca lista standardów, ale chciałem podkreślić ich istnienie i zapewnić pewne wyjaśnienia.
Wniosek
Rozumiejąc zasadę działania akumulatorów litowo-jonowych i biorąc pod uwagę takie czynniki, jak jakość ogniw, konstrukcja opakowania i solidny system zarządzania akumulatorami, możemy zwiększyć bezpieczeństwo i niezawodność akumulatorów. Przestrzeganie odpowiednich norm i przeprowadzanie dokładnych testów dodatkowo przyczynia się do bezpiecznego i wydajnego wykorzystania akumulatorów litowo-jonowych.
Dzięki ciągłemu postępowi technologicznemu i skupieniu się na bezpieczeństwie akumulatory litowo-jonowe będą nadal odgrywać znaczącą rolę w zelektryfikowanym świecie, zasilając różne zastosowania, a jednocześnie ograniczając ryzyko.
Powiązane artykuły:


