Key Takeaways:
- Prevalence and Operation: Lithium-ion batteries are widely used for their high energy density and no memory effect. They operate through the reversible movement of lithium ions between the cathode and anode.
- Failure Causes: Common reasons for battery failure include organic electrolyte evaporation, separator melting, oxygen release, uncontrolled charging, rapid charging at low temperatures, complete discharge, and manufacturing defects.
- Prevention Strategies: Ensuring battery longevity requires the use of high-quality cells, effective battery pack design, and a reliable battery management system (BMS).
- BMS Importance and Features: A BMS is crucial for monitoring voltage, temperature, and cell balance. It should comply with safety standards like UL 1642 and IEC 62133 for cells, and UL 991 or UL 1998 for BMS software.
Lithium-ion batteries are all around us, powering our smartphones, laptops, electric vehicles, and renewable energy storage systems.
In this post, we’ll explore the basics of these batteries, including how they work, their benefits, common failure causes, and prevention methods.
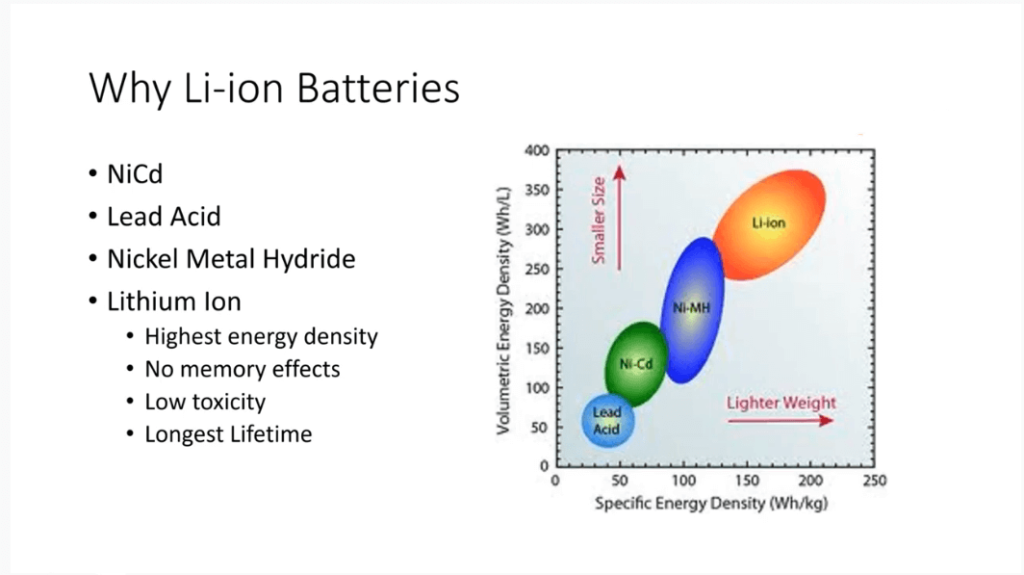
Why Use Lithium-ion Batteries?
Lithium-ion batteries have become popular due to their high energy density. They are superior to lead-acid, nickel-cadmium, and nickel-metal hydride batteries in both volume and mass-based energy density.
The transition from nickel-cadmium to nickel-metal hydride batteries has led to the widespread use of lithium-ion batteries. These batteries not only offer the highest energy density but also have no memory effect. This means their capacity is not affected by a full or partial charge or discharge.
Besides, Lithium-ion batteries have low toxicity. Especially the lithium iron phosphate batteries, they don’t contain heavy metals like cobalt. Also, they have a longer lifetime than alternative chemistries, ensuring reliability in various applications.
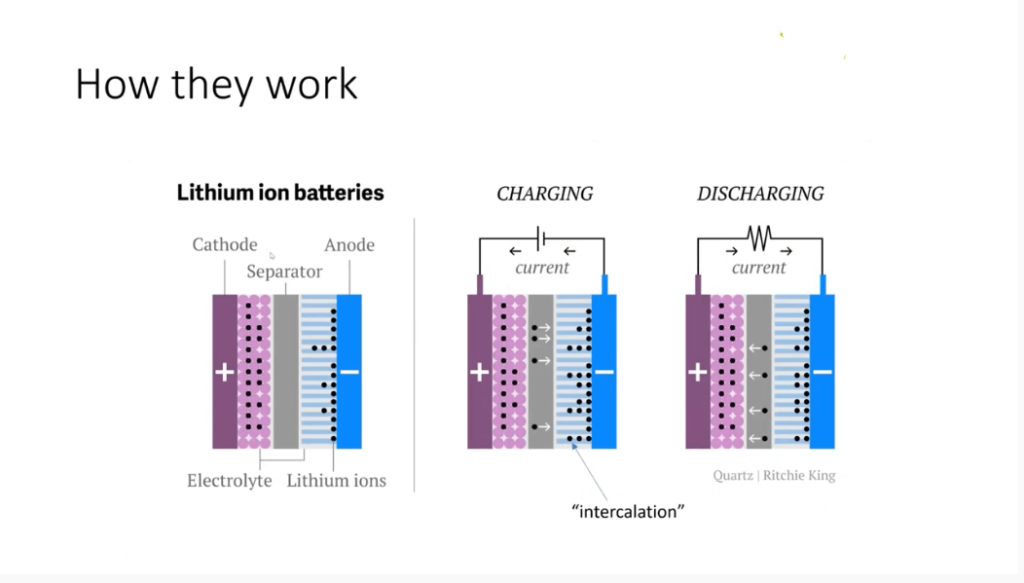
How Lithium-ion Batteries Work?
To understand the safety concerns surrounding lithium-ion batteries, it’s important to grasp how they work. Like any electrochemical cell, a lithium-ion battery consists of a cathode and an anode. The cathode usually contains a lithium salt, such as lithium oxide or lithium phosphate, while the anode is typically made of graphite.
When you charge a lithium-ion battery, the lithium ions (represented by black dots) move from the lithium oxide salt to the graphite anode. This movement, known as intercalation, does not involve direct interaction between the ions and the electrons. Instead, the electrons flow from the cathode to the anode, where they react with the carbon in the graphite.
It’s worth mentioning that unlike lithium metal batteries, which are non-rechargeable, lithium-ion batteries allow for the reversible intercalation of lithium ions. This breakthrough innovation awarded John Goodenough and Stan Winningham the Nobel Prize in Chemistry. The lithium ions undergo diffusion through an organic electrolyte fluid, enabling their back-and-forth movement between the anode and cathode.
In the following part, we will dig more into the organic electrolyte and its function in aiding the smooth operation of lithium-ion batteries.
LCO, LMO, NCA
Let’s start by discussing the cathode and the lithium salts usually used in lithium-ion batteries. The first one we’ll study is lithium cobalt oxide, which is widespread in laptops, power tools, and cell phones. When the battery discharges, the lithium separates from the lithium cobalt oxide, releasing an electron that travels through the charger to the anode. This procedure leaves cobalt oxide on the cathode.
Another salt used as a cathode material is lithium manganese oxide. This type of cathode was utilized in the Nissan Leaf and can also be found in various Tesla models like the Model S, Model 3, and Model X.
Lastly, we have lithium nickel cobalt aluminum oxide, which delivers the highest energy capacity per mass and volume.
Causes Of Li-ion Battery Failure
In order to prevent Li-ion battery failures, it’s important to be aware of the factors that can lead to such issues. Let’s take a closer look at some common causes.
Organic Electrolyte Evaporation
If a Li-ion battery gets too hot, the organic electrolyte inside can evaporate. This evaporation increases the pressure and temperature within the cell. As a result, the battery may bulge, indicating the presence of hazardous conditions.
Separator Melting
Li-ion batteries typically use a separator made of polyethylene or polypropylene. When exposed to temperatures around 80 degrees Celsius (170-180 degrees Fahrenheit), this separator can melt. The melting of the separator allows the anode and cathode to come into contact, leading to an internal short circuit and generating additional heat.
Oxygen Release and Uncontrolled Reactions
When a Li-ion battery reaches high temperatures, the oxygen present in cathode materials like lithium cobalt oxide, lithium manganese oxide, or lithium nickel cobalt aluminum oxide can be released. This released oxygen can react with the evaporated electrolyte, causing uncontrolled chemical reactions. The continuous short circuit further exacerbates the situation, making it crucial to address it promptly.
Uncontrolled Charge
Overcharging the battery or subjecting it to an uncontrolled charge can lead to the formation of lithium metal on the anode. Excess electrons combine with lithium ions, forming dendrites that grow through the electrolyte and into the cathode. These dendrites can create internal short circuits, posing serious risks.
Rapid Charging and Low Temperatures
Charging the battery at very high currents or low temperatures can hinder the movement of lithium ions into the anode. Consequently, an excess of electrons may accumulate on the anode, causing lithium metal plating and potential internal short circuits.
Complete Discharge
Avoid fully discharging a lithium-ion cell. Over-discharging can cause the copper collector on the anode to dissolve into the electrolyte. When recharging, the copper may reform, but not in its original foil-like structure. This can lead to copper plating and result in an internal short circuit.
Poor Cell Production and Contamination
Li-ion battery failures can also occur due to production flaws or the presence of impurities during manufacturing. These impurities can introduce contaminants or particulates into the battery, leading to internal short circuits or unwanted reactions that accelerate capacity degradation.
By understanding and addressing these causes of Li-ion battery failure, we can work towards enhancing battery safety, reliability, and longevity in various applications.
Preventing Battery Failure
Preventing issues in the battery industry is crucial for its continued growth and success. There are three key steps that can be taken to effectively minimize the occurrence of problems.
First and foremost, ensuring the quality of battery cells is of utmost importance. With the rapid expansion of the industry, numerous cell manufacturing facilities, particularly in China, have emerged. It is crucial to carefully select high-quality cells from reputable manufacturers. Some facilities boast top-of-the-line, high-tech automated processes, while others may not meet the same standards. The choice of cell quality directly impacts overall performance and reliability.
The design of the battery pack also plays a vital role in preventing incidents. Battery packs consist of multiple cells arranged in series and parallel configurations, creating the desired voltage and current capacity. When designing a pack, it is essential to consider effective heat dissipation in case of unforeseen events. Understanding how the pack will respond to potential cell issues is vital for maintaining safety. Furthermore, if the system requires delivering substantial amounts of current, ensuring efficient distribution through reliable contacts and circuit boards becomes paramount.
At the crux of it all is the battery management system (BMS). This device serves as the guardian of the battery, continuously monitoring voltages, currents, and temperatures to ensure the cells operate within safe limits. In any lithium-ion battery pack, the presence of an integrated or external BMS is critical for safeguarding the cells. The BMS not only ensures safety but also enhances the longevity of the batteries. Given that lithium-ion batteries can outlast conventional storage devices by a significant margin, it becomes imperative to prioritize their protection for long-term usage.
Preventing issues in the battery industry necessitates careful attention to cell quality, pack design, and the implementation of a reliable battery management system. These collective measures contribute to the overall safety and durability of lithium-ion batteries, enabling the industry to thrive while minimizing potential risks.
Importance Of Battery Management Systems
The battery management system (BMS) plays a crucial role in monitoring and controlling the voltages, currents, and temperatures of a battery. Its primary function is to ensure that the battery operates within safe limits. If the BMS detects any abnormalities or exceeds cell limits, it has the capability to interrupt the charging or discharging process.
In simpler terms, the BMS keeps an eye on the battery’s vital signs. It constantly checks the voltage levels, current flow, and temperature to make sure everything is functioning properly. If it detects any issues, such as excessive heat or irregular voltage, it can take action to protect the battery.
One of the key tasks of the BMS is to prevent overcharging or overdischarging of the battery. Overcharging can cause damage to the battery cells and reduce their lifespan, while overdischarging can lead to performance degradation. The BMS ensures that the battery receives the appropriate amount of charge and prevents it from getting too full or too empty.
Think of the BMS as the battery’s guardian. It is always on watch, ready to step in and protect the battery from potential harm. By monitoring and regulating the battery’s parameters, the BMS helps to extend its overall lifespan and maintain optimal performance.
What Features Should Be Present On A BMS?
We want to share my opinion on the minimum requirements for a BMS to ensure the protection and longevity of the battery pack.
Firstly, voltage protection is essential. It’s crucial to prevent overcharging and overdischarging of the battery. We need to maintain a safe voltage range to avoid damaging the cells and maximize their lifespan. By the way, we should also consider preventing the pack from delivering currents that exceed its capacity, not just at the cell level but for the entire pack as well.
Temperature protection is another vital aspect. When temperatures rise too high, it can lead to potential risks and failures. Therefore, having mechanisms in place to monitor and control high temperatures is crucial. Similarly, it’s important to have low-temperature charging protection to prevent issues like lithium metal plating on the anode due to excessively cold conditions.
Furthermore, one useful feature, although not absolutely necessary, is the ability to balance the cells within a series. Cells in parallel naturally share current and voltage, but cells in series do not. To maintain a uniform state of charge (SOC) among the cells, a balancing mechanism or extra current sharing capability is required.
Finally, while we didn’t discuss specific standards for third-party testing, it is worth mentioning that there are existing standards that third-party test labs can use for compliance assessment.
Standards
There is often confusion regarding the different listings for cells, battery packs, and battery management systems. Let’s clarify things a bit. Lithium-ion cells can be tested and listed according to UL 1642 or IEC 62133 standards.
Battery packs, on the other hand, have their own listings. They can be listed under either UL 2050 or UL 1973, both of which require compliance with UL 1642 as a prerequisite. It’s important to note that UL 1642 itself is not a pack listing but rather a prerequisite for these pack listings.
In an attempt to create a listing that applies to both cells and packs, the IEC introduced IEC 62133. However, it’s worth mentioning that battery management systems (BMS) also have their own separate listings.
For hardware, the BMS can be listed to UL 991, while for software, it can be listed to UL 1998 or IEC 60730-1. It’s important to note that UL 991 and UL 1998 are not prerequisites for UL 2054 or UL 1973 listings.
However, if your BMS is not listed to these standards, you will need to conduct testing using fault conditions to ensure that even in the event of a failure, a dangerous situation is not created.
It’s important to remember that this is not an exhaustive list of standards, but I wanted to highlight their existence and provide some clarification.
Conclusion
By comprehending the working principle of lithium-ion batteries and considering factors such as cell quality, pack design, and a robust battery management system, we can enhance battery safety and reliability. Adhering to relevant standards and conducting thorough testing further contribute to the safe and efficient utilization of lithium-ion batteries.
With continued advancements in technology and a focus on safety, lithium-ion batteries will continue to play a significant role in our electrified world, powering various applications while mitigating risks.
Related Articles:


